Odetiglucan
A Systemically Administered Innate and Adaptive Immune Modulator
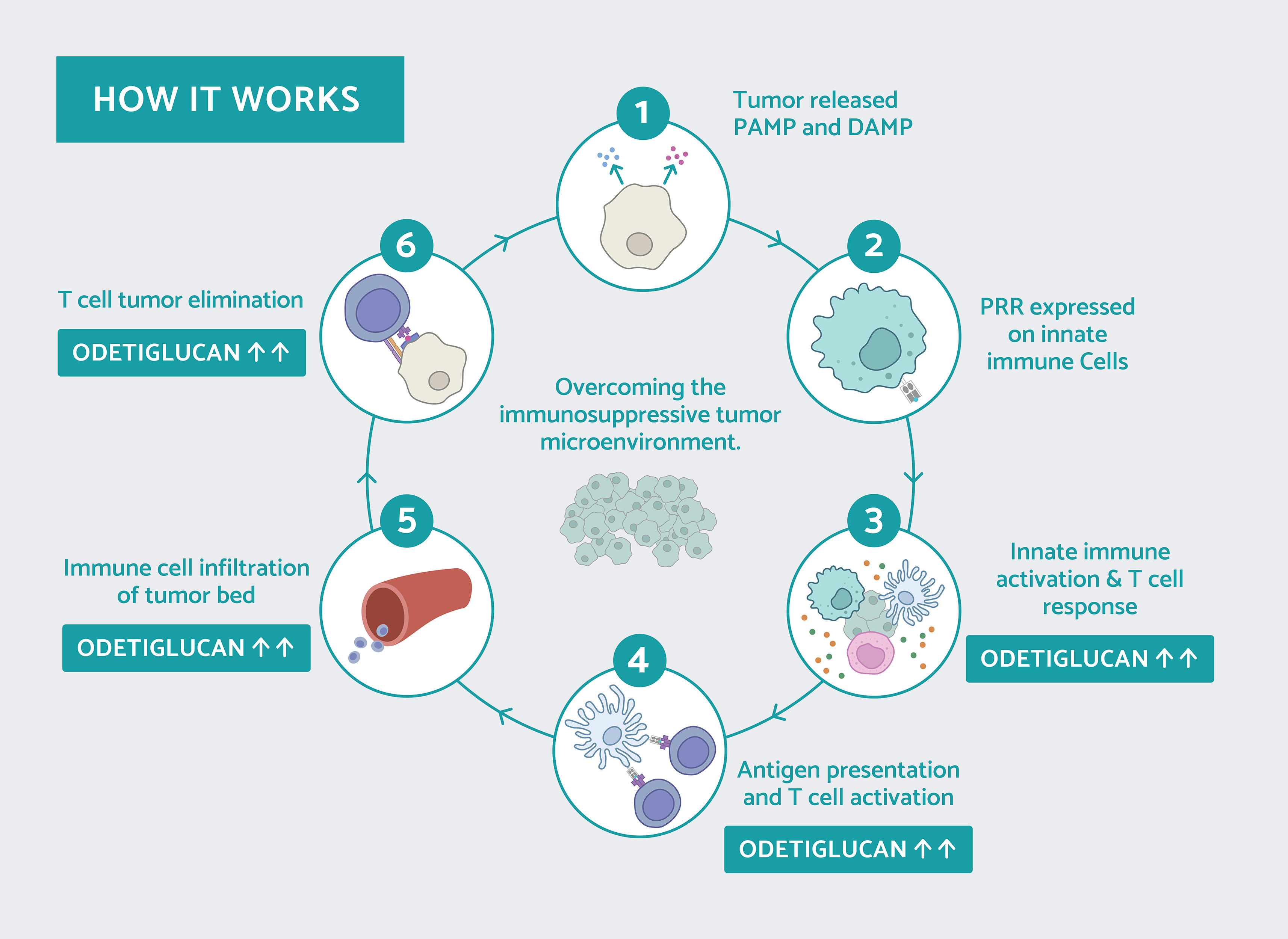
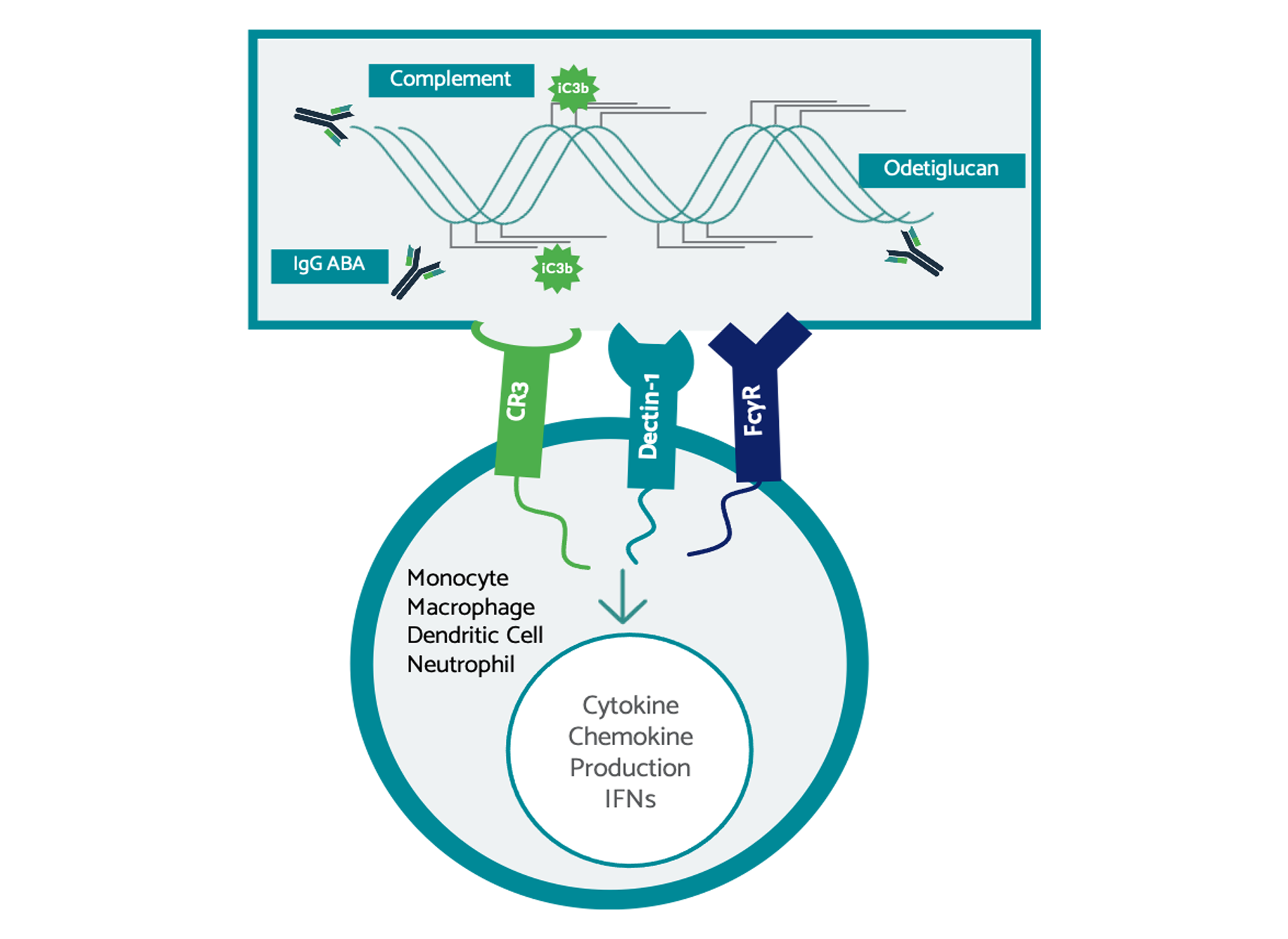
Odetiglucan, our immune modulator therapeutic candidate, is systemically administered and has been observed to modulate both the innate and adaptive immune systems.
Odetiglucan is a pathogen-associated molecular pattern (PAMP) which is designed to enhance innate immune functions, including cytotoxic effector mechanisms, to reverse immunosuppression and facilitate cross-talk with the adaptive immune system. We have observed clinical benefits of odetiglucan as a monotherapy and in combination with multiple standard of care therapies in different tumor types.