Odetiglucan
Clinical Development of Odetiglucan
----------
Odetiglucan has also demonstrated increased response rates and disease control rates in patients with liver metastasis across varied tumor histologies, with multiple therapeutic classes. Odetiglucan will be evaluated in a Phase 2 investigator initiated clinical trial further exploring this mechanism in combination with an immune checkpoint inhibitor in patients with colorectal cancer liver metastasis.
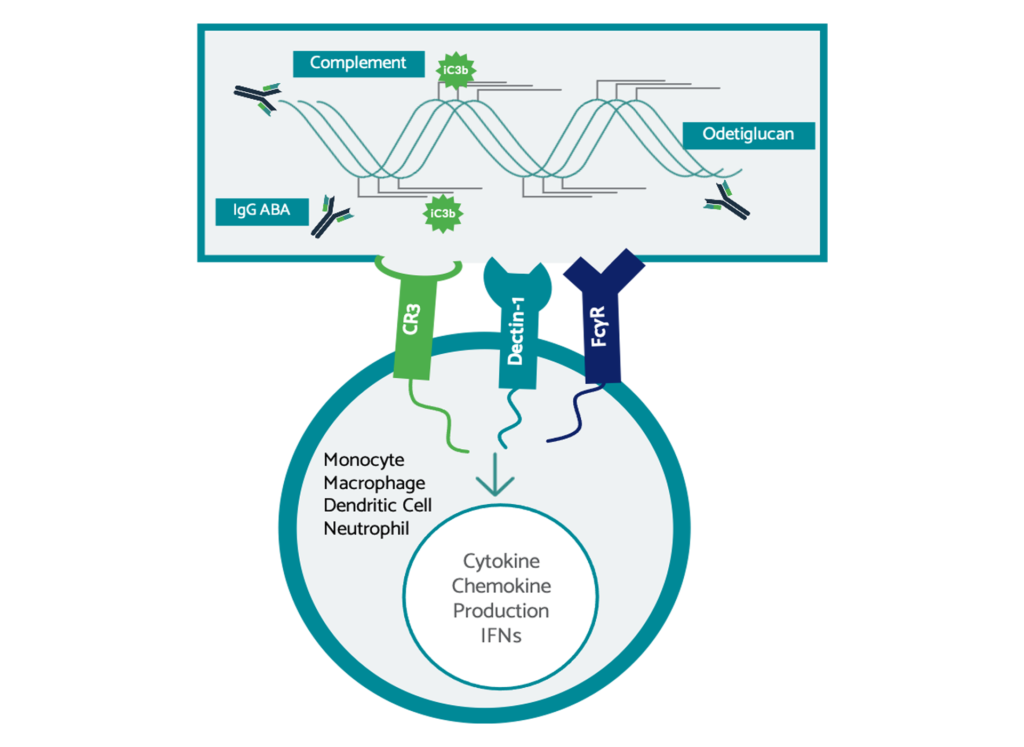
The Odetiglucan Immune Complex
Odetiglucan, our immune modulator therapeutic candidate, has been observed to modulate both the innate and adaptive immune systems. Odetiglucan is designed to form an immune complex in vivo via three receptors: Dectin-1, FcɣR, and CR3 and has been observed to drive anti-tumor immunity.
Unlike many other PRR agonists (cGAS-STING, TLR, etc.), Odetiglucan has been observed clinically to be generally well-tolerated and systemically administered due to its non-inflammatory, chemokine-centric innate immune activation. As a result, we believe that Odetiglucan is not limited to intratumoral injection and has been observed not to be reliant on abscopal effect for systemic change.
We have observed that Odetiglucan drives anti-tumor immunity by reprogramming the immunosuppressive tumor microenvironment, enhancing antigen presentation, priming a de-novo T cell immune response, and inducing trained immunity, driving a memory