Our Science
Our Science
Our Science
Precision Oncology is Incomplete
Integrated Stress Response (ISR) represents a unique non-genomic therapeutic target in cancer
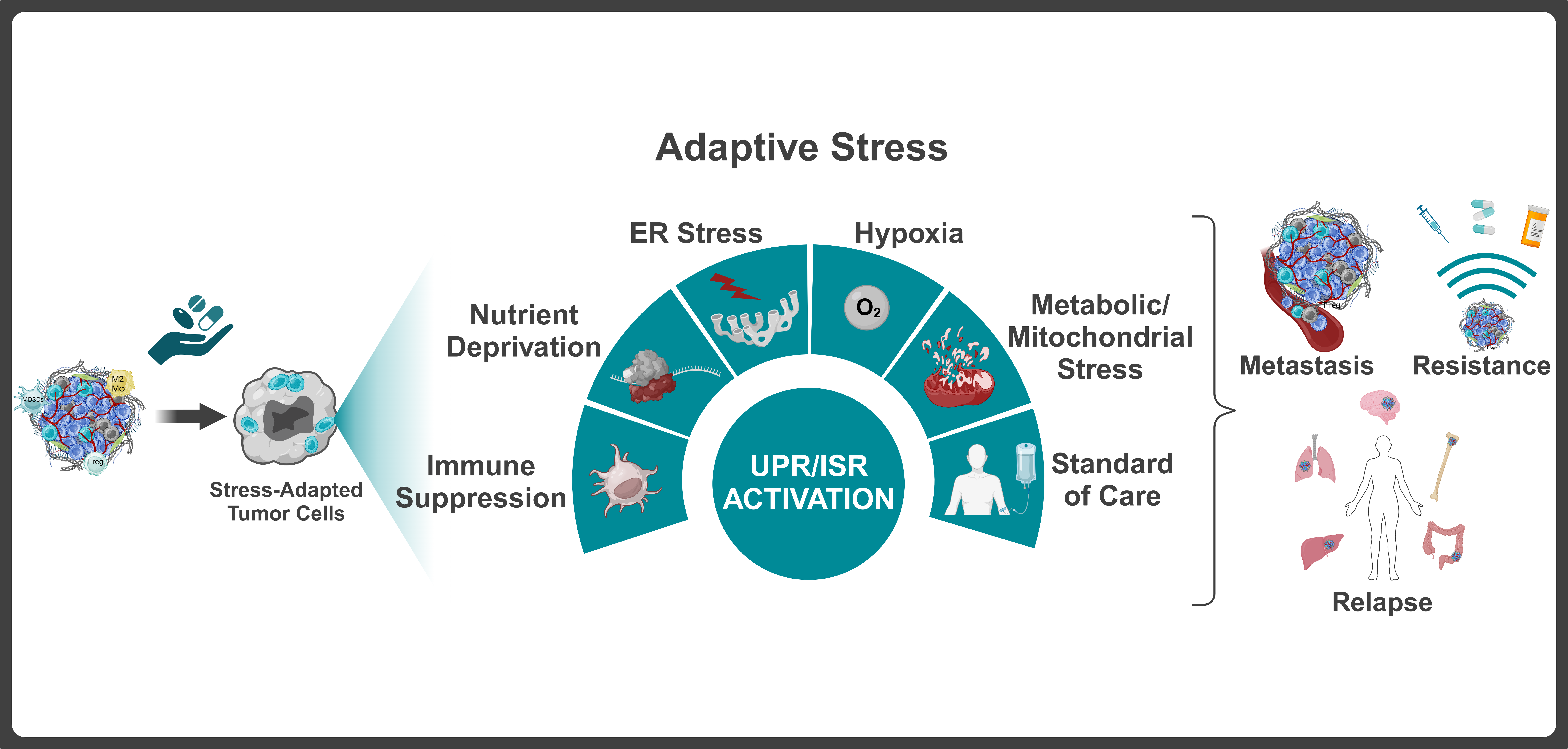
The integrated stress response is a signaling pathway activated in cancer cells in response to intrinsic stress signals such as oncogenic drivers and ER stress, and external microenvironmental imbalances including nutrient deprivation and hypoxia. This adaptive stress response plays a critical role in dysregulated cancer growth, immune escape, and cancer metastasis. Additionally, a number of targeted therapies in oncology targeting precise molecular aberrations, can also induce or exacerbate stress leading to acquired resistance and ultimately, cancer relapse.
Therapeutic Approaches Targeting the ISR Spectrum
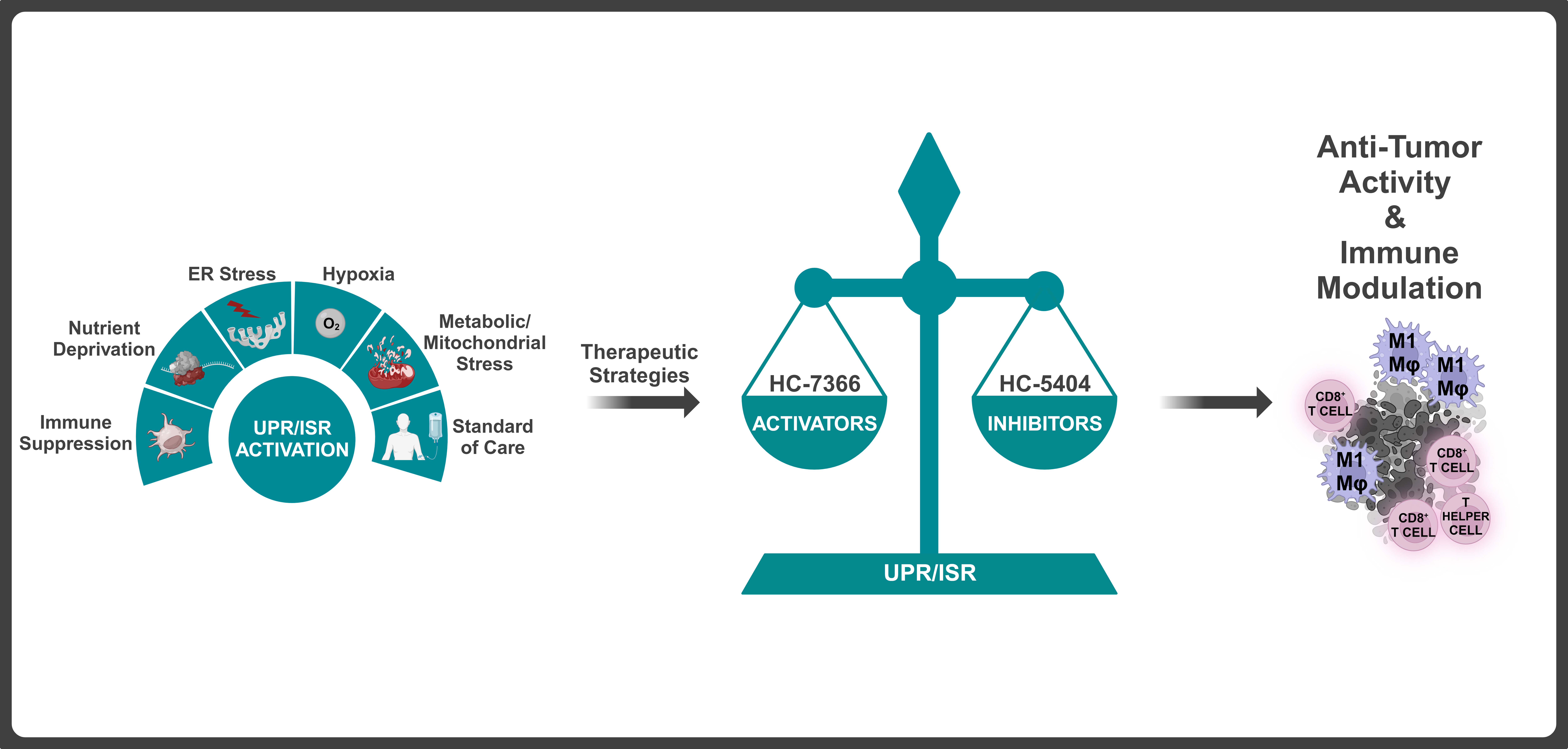
Activation of ISR is suggested to play a dual role in cell fate decisions. While acute activation of ISR promotes survival, prolonged activation of ISR induces apoptosis. Thus, either inhibition or activation of the ISR can disrupt the homeostatic equilibrium of the cancer cells. Our programs span the ISR spectrum by:
Targeting the adaptive survival mechanism of ISR, the unfolded protein response (UPR) by inhibiting the kinase PERK by HC-5404
Inducing cell-death mechanisms via hyperactivation of the ISR kinase, GCN2 by HC-7366